What is CLIA In Medical billing? - Every Details Covered
- Updated Date Oct 7, 2025
- Medical Billing
- Follow
Clean claims come from good daily habits. CLIA is one of those habits. It shows your testing site is approved to run the tests you bill. It also helps payors process your claim without extra questions.
If your team runs in-office tests like flu, strep, or COVID antigen, CLIA is part of your workflow every day. Pick the right certificate. Keep it active. Put the CLIA number in the correct claim field. When these steps line up, More first-pass approvals and less follow-up. In this blog we will cover:
- What is CLIA in medical billing?
- What Is A CLIA Number?
- Types of CLIA certificates
- When do claims need a CLIA number?
- How do you obtain a CLIA certificate?
- Where do you put the CLIA number on claims?
- Common CLIA Denial Reasons and Ways to Prevent Them
- Conclusion
What is CLIA in medical billing?
CLIA stands for Clinical Laboratory Improvement Amendments. It is a federal program that sets rules for all human lab testing in the United States. In medical billing, CLIA matters because payors use it to confirm that the place doing the test is approved to run that test.
What Is A CLIA Number?
A CLIA number is the unique ID that the government gives to a specific testing site, like your clinic’s lab room. It links your location to your current CLIA certificate, which shows the level of testing you are allowed to perform.
Think of it as your lab’s license plate. Payors use the CLIA number to verify three things before paying a claim: the place that performed the test, the certificate type on file, and whether that certificate was active on the date of service.
Types of CLIA certificates
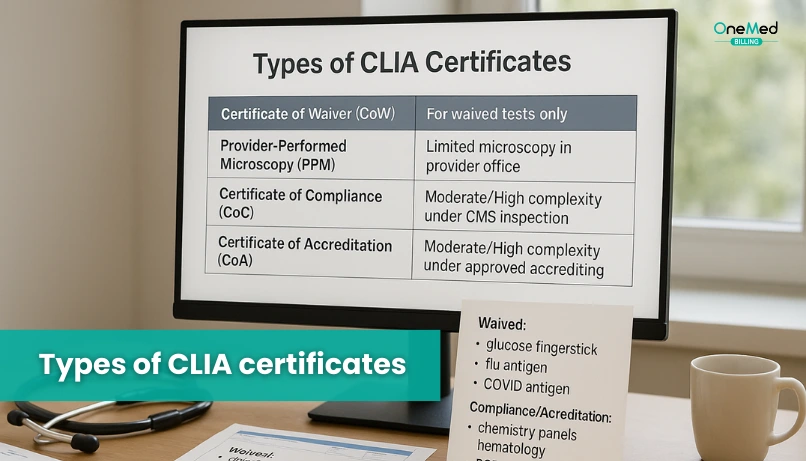
Below are the five CLIA certificate types. Each one sets clear limits on what testing a site can perform.
Certificate of Waiver
For sites that only run waived tests such as basic flu, strep, or COVID antigen tests. It is simple to obtain and covers low-risk, easy-to-use methods.
Certificate for Microscopy
Also called Provider Performed Microscopy Procedures. It allows qualified clinicians to perform a short list of microscopy tests at the point of care, like wet mounts, within defined rules.
Certification of Registration
A temporary status that lets a new or expanding lab begin moderate or high complexity testing while it awaits its first inspection. It is an interim step, not a final approval.
Certificate of Compliance
Issued after a lab passes inspection by the state or CMS. It confirms the lab meets CLIA requirements and may perform nonwaived testing at the approved complexity level.
Certificate of Accreditation
Granted to labs that are inspected and accredited by a CMS-approved body such as CAP, COLA, AABB, ASHI, or the American Osteopathic Association. It shows the lab follows an external quality program that meets federal standards.
When do claims need a CLIA number?
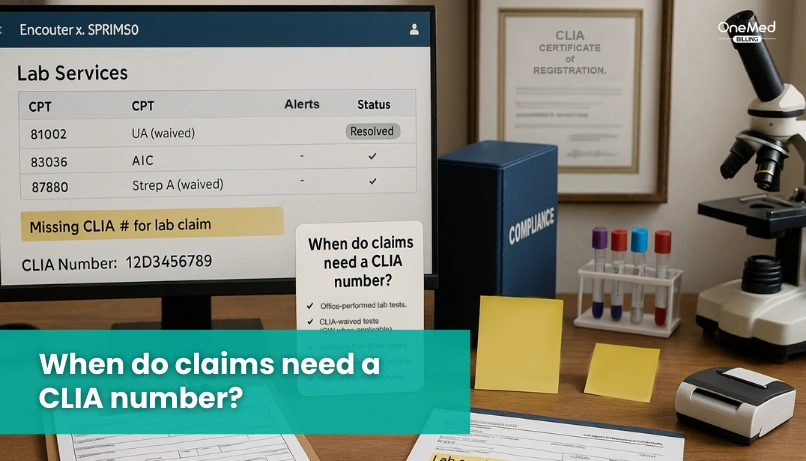
You must include your CLIA number when you bill for lab tests your site performs on human specimens. Payors use it to confirm your location is approved for the level of testing done on the date of service.
If your clinic runs point-of-care tests, the CLIA number is usually required. This includes common waived tests done in offices, urgent care, and outpatient clinics. It also applies when you perform moderate or high complexity tests under the right certificate.
If a reference lab performs the test and bills the payor directly, you do not add your CLIA number. If you bill as the performing site, you must use a valid CLIA number for your location.
Make sure the number you use matches three things. It must match the site that performed the test. It must match the certificate level needed for that code. It must be active on the date of service.
How do you obtain a CLIA certificate?
Getting a CLIA certificate follows a simple, ordered process that most clinics can complete without trouble. Use the steps below to obtain a CLIA certificate.
1) Decide what testing you will perform
List the tests you plan to run in the office. Choose the certificate type that fits those tests. Most clinics start with a Certificate of Waiver for point-of-care tests.
2) Complete the CLIA application
Fill out CMS Form 116 with your legal business name, tax ID, NPI if you have one, physical testing address, and the certificate type you need. Each testing location needs its own application.
3) Send the form to your State Agency
Submit the form to your state’s CLIA contact. They will review the application and tell you the fee amount and how to pay.
4) Pay the CLIA fees
You will receive a fee notice. Pay it in full. Fees vary by certificate type and test volume.
5) Receive your CLIA number
After payment is processed, you will get your CLIA number and a certificate or a Registration letter if you requested moderate or high complexity. Registration lets you start testing while you wait for an inspection.
6) Prepare for inspection if you are nonwaived
If you chose moderate or high complexity, prepare for an inspection by the state or an approved accrediting body. Once you pass, you will receive a Certificate of Compliance or Certificate of Accreditation.
7) Certificate is issued
Once approved, your site receives its active CLIA certificate. If you are waived, you get a Certificate of Waiver. If you are moderate or high complexity, you receive a Certificate of Compliance or Certificate of Accreditation after passing inspection.
Where do you put the CLIA number on claims?
Place your CLIA number in the claim fields payors check for lab certification. Use the quick map below to see exactly where to enter it on paper and electronic claims.
Paper CMS-1500
- Put the CLIA number in Box 23.
- Enter the 10-character format without spaces, for example 12D3456789.
- Make sure the Service Facility Location reflects the site that performed the test.
Electronic 837P (professional)
- Send the CLIA in Loop 2300, REF segment, with qualifier X4.
- Example structure: REF*X4*12D3456789~
- Include the correct Service Facility segment for the performing site.
Institutional or hospital claims (837I / UB-04)
- When required, submit the CLIA in the claim-level REF, qualifier X4.
- Follow the hospital’s billing policy for the form locator that your payor specifies.
Common CLIA Denial Reasons and Ways to Prevent Them

A few small errors with CLIA can stall your payments. Before you submit any lab claim, make sure the CLIA number is in the right place, the certificate is active, and the test matches your certificate level. Use the quick checks below to prevent avoidable denials.
1. CLIA number missing or in the wrong field
The claim goes out without the site’s CLIA number, or it is entered where the payor does not read it. The claim pends or denies.
How to prevent: Enter the CLIA in Box 23 on paper claims or in the 837P REF with qualifier X4, and validate before submission.
2. CLIA certificate expired or does not match the test complexity
The certificate on file is past its end date, or the test needs a higher level than your certificate allows. Payors reject because the site is not eligible for that service.
How to prevent: Track renewal dates, keep the certificate active, and match your test menu to the approved certificate level.
3. Modifier QW is missing when required
Many waived tests need QW to show they are CLIA waived. If QW is missing on a code that requires it, the claim will likely be denied.
How to prevent: Maintain a current list of QW-required codes and build front-end edits so QW is added when needed.
4. Billed under the ordering provider instead of the performing site
The claim lists the ordering clinician as the one who performed the test. Payors cannot verify the correct CLIA number tied to the performing location.
How to prevent: Bill under the performing site with the correct service facility details and matching CLIA number.
5. Service facility does not match the CLIA site
The address on the claim points to a different location than the one that ran the test. This creates a mismatch with the CLIA record and can trigger a denial.
How to prevent: Use the exact testing location address that matches the CLIA record for the date of service.
6. Wrong or outdated CLIA format
The number is mistyped or sent with spaces or extra characters. Edits fail, and the claim is rejected.
How to prevent: Use the 10-character CLIA format with no spaces and confirm the value with a pre-submit check.
Conclusion
CLIA rules are simple when you break them down. Use the right certificate, keep it active, and place the CLIA number in the correct claim field. Add QW when a code needs it and always bill under the performing site.
A few pre-submit checks before submission can stop most denials. For help building front-end edits, keeping a current QW list, and aligning service facility details with the correct site, partner with OneMed Billing to set up a workflow your staff can trust.
Frequently Asked Questions
Find quick answers to common questions about this topic, explained simply and clearly.
What is CLIA, and what is its purpose?
Federal program that certifies labs and sets quality rules so test results are accurate and reliable.
What is a CLIA denial?
Claim rejection caused by missing, wrong, or mismatched CLIA details.
What is the CLIA number on a claim?
10-character ID for the testing site. On CMS-1500 use Box 23. On 837P use REF X4.
How do you get a CLIA number?
Pick your certificate type, file CMS Form 116 with your State Agency, pay the fee, then receive your number. Nonwaived labs complete an inspection.